


|
 |
|
|
#1 |
|
Registered Member
Join Date: Sep 2007
Location: NY, NY
Posts: 105
|
So in my search for a new skimmer I've learned a bit about the theory of skimming. There is a tradeoff between efficiency and volume, in that the organic concentration of skimmate is higher if there is less skimmate (dry skimming), however the total organic content removed is higher in wetter skimming. Of course the wetter skimming the more often you have to empty the collection, and the more salt you lose to skimming.
I read in one article that wet skimming and top off with salt water is a good way to accomplish water changes, it would also minimize some of the hassle of water changes. Clearly you'd have to make sure you maintain the salinity of the water, and the value would be different for every person. If your skimmate is low in salt concentration you would need less salty top off and if it is high in salt you would need saltier top off. The article even mentioned some people having skimmate saltier than the bulk water of the tank in which case your top off would need to be hypersalic (is that even a word). Because you would be removing water that had a higher concentration of yuckiness (definitely a word) than the bulk water my theory is that you wouldn't need to meet the 10% per week rule (though more is usually better). A water container could easily collect wet skimmate, given that these are available in various sizes (a quick check shows me easily up to 6 gallons), you could empty it twice a week and do a 10% "change on a 120 gallon tank. Of course I would rather a smaller waste collection bin and empty it more frequently. When going out of town for a few days I would likely just set my skimmate to dryer and not worry about it at all. The principal problem here is matching salt removed and salt added. Does anyone have a good method. I see that the aquacontroller pro has a conductivity measurement, in which case you could have two top offs, one with hyper saline water and one with RO water. If the conductivity indicates less than 24 parts per thousand add salt water, if it indicates more than 25 or the water level is too low add fresh water, of course you would need a dosing pump for the salt water. You could set up a top off of RO water, and leave quite a bit of space in the sump above where the top off filled (so as to not overflow by adding salt water). The principal benefits of the system would be 1) increased stability, in terms more constant water chemistry and constant temperature. More constant water temperature because you never have to attempt to match the temperature of water change water with the temperature of water in the tank. 2) reduced work, its easy to make saturated salt solution, which contains 10 times the concentration needed (wikipedia sea water and sodium chloride), so for 10 gallons of water change you need 9 gallons fresh water and 1 gallon saturated salt water. I'm totally trying this, maybe not now, but in a few years. If anyone has the capability and the desire to try this please let us know how it works. Or if like always I've missed the proverbial boat and you've been doing this I'd love to know how it's been working. |
|
|

|
|
|
#2 |
|
Registered Member
Join Date: Jul 2003
Location: Winter Park, FL
Posts: 2,707
|
I'm a big fan of wet skimming. In practice it is more of a long term salinity drift than anything that requires a complicated solution.
BTW you can't just make a 10x concentrate of saltwater. You will get precipitates. |
|
|

|
|
|
#3 |
|
Registered Member
Join Date: Sep 2007
Location: NY, NY
Posts: 105
|
Discocarp, so then I assume you are also doing regular water changes?
You're worried about other things falling out of solution before NaCl? 10x is what you could do if it were pure NaCl (hence me citing where I got the info from). Even if it is the case that something else precipitates before NaCl, I feel confident you could hit 5x. Really there's not much else in seawater besides magnesium. Also there's a difference between wet skimming and what I'm discussing, I'm talking about on the order of gallons (maybe 1 per 70 gallons per day) of skimmate per day. |
|
|

|
|
|
#4 |
|
Registered Member
Join Date: Jul 2003
Location: Winter Park, FL
Posts: 2,707
|
Yep, I do regular water changes. When I get busy I just topoff with saltwater occasionally.
What will precipitate out is calcium carbonate. Try it sometime. Put 10x the normal salt in a gallon of rodi. It will precipitate the calcium and alk out of solution. Dilute it with 9 more gallons of water, mix, then test ca+alk. It will be very low. This is also why you never mix salt water by adding water to salt instead of adding salt to water. |
|
|

|
|
|
#5 |
|
Reef Chemist
 Join Date: Apr 2001
Location: Arlington, Massachusetts
Posts: 86,233
|
I've been using wet skimming (a few gallons per day) and automatic salt water additions to replace the skimmate as all or part of my automatic water change regimen. I think it is a fine idea, but can be a little tricky to fully automate as the skimmate production varies over time.
Even if it is the case that something else precipitates before NaCl, I feel confident you could hit 5x. Really there's not much else in seawater besides magnesium.  And your body is not much more than water.  Overconcentrating seawater is a poor plan, IMO, for the reason given above.
__________________
Randy Holmes-Farley Current Tank Info: 120 mixed reef |
|
|

|
|
|
#6 |
|
Registered Member
Join Date: Sep 2007
Location: NY, NY
Posts: 105
|
Not do discount the objections, they are the same worries I have myself, but before seeing an experiment I'm not prone to believe the objections in full. There is some point at which water will be saturated with "salt" which is in all likelihood much greater than the concentration that we use.
FYI salt to water or water to salt doesn't make any difference. Despite the fact that I am a chemistry graduate student, I am more a biocomputationalist myself and therefore not in a good position to answer the question "what is the multi-ionic concentration of saturation in water?". After discussion with someone better posed to answer the question I find that it's a much harder question than it sounds, impossible analytically, difficult numerically and trivially easy experimentally. The best way to determine the maximal concentration is to add a known quantity of salt to a container, and then add water until the salt can be dissolved. The advantage of this is it is easier to overshoot saturation when adding solid to liquid than when adding liquid to solid. I could try to work it out later today, but it would only be as accurate as volume of salt to volume of water, I'd much rather do it with a gram balance. Randy, the advantage of the system discussed is that it accommodates varying production of skimmate. Though I'm glad to hear it's been working for you. Watch out this and dual photoperiods are totally going to be incorporated in the 2013 september tank of the month. |
|
|

|
|
|
#7 | |
|
Registered Member
Join Date: Jul 2003
Location: Winter Park, FL
Posts: 2,707
|
Quote:
I dare you to add enough salt to make 50 gallons to a container and then let ro fill it slowly. You will end up with saltwater with little ca/alk and a lot of white precipitate (calcium carbonate) on the bottom. |
|
|
|

|
|
|
#8 |
|
Registered Member
Join Date: Sep 2007
Location: NY, NY
Posts: 105
|
Have you actually tried this, adding a liquid to a solid? Perhaps you should give it a little more time when mixing? I'm happy to try myself later.
|
|
|

|
|
|
#9 |
|
Reef Chemist
 Join Date: Apr 2001
Location: Arlington, Massachusetts
Posts: 86,233
|
but before seeing an experiment I'm not prone to believe the objections in full.
 This is not new ground. No need to experiment. What happens when seawater is concentrated has been very well studied and published in the scientific literature. There is a section detailing it in Chemical Oceanography by Frank Millero. This is not new ground. No need to experiment. What happens when seawater is concentrated has been very well studied and published in the scientific literature. There is a section detailing it in Chemical Oceanography by Frank Millero.  In salt mixes with higher than NSW levels of pH, alkalinity, and calcium, such precipitation may happen even earlier than for normal seawater. FWIW, I discuss precipitates in many settings here: What is that Precipitate in My Reef Aquarium? http://reefkeeping.com/issues/2005-07/rhf/index.htm from it: When salt mixes are dissolved, there exist local regions where the salt concentration is very high. In those local regions, the calcium and alkalinity must also be very high. In fact, as seawater is concentrated by evaporation, there is a well-established series of minerals that precipitate as the salinity increases. In this series, calcium and magnesium carbonate are the first to precipitate, appearing at a specific gravity of about 1.140, which is about a 50% solution of salt in water. Such conditions may well exist on the bottom of a saltwater reservoir as the salt is dissolving.
__________________
Randy Holmes-Farley Current Tank Info: 120 mixed reef |
|
|

|
|
|
#10 |
|
Registered Member
Join Date: Sep 2007
Location: NY, NY
Posts: 105
|
That's great 1.140 is 5.6x concentration of salt, (1.140-1)/(1.025-1) so not 10x but 5.6x. Still this has the benefit of meaning instead of a 56 gallon reservoir you can get by on a 10 gallon reservoir.
I believe the 50% that they refer to is 50% saturation of an NaCl solution (i.e. saturated salt water). Although in table 2.14 it seems that the first precipitate is gypsum (CaSO4) at 3.62x concentration, not that a tank requires sulfate in large quantities, nonetheless tis better to not screw too much with nature. If one were interested one could find the amount of calcium that you could obtain if you were willing to let gypsum precipitate, in which case you could push the concentration all the way up to 9.8x, if you're not losing much calcium to gypsum this could be plausible (and if one has a calcium reactor, and is adding calcium that way...). Additionally all of this assumes that the salt mix mirrors the concentrations of the ocean, and by ocean I assume Millero means middle of pacific and 25 C or 77 F, so you might be able to squeak a little more out at 80 F but I think it's far more reasonable to just play it safe and use a bigger tank. I still think this is a good idea, my reservoir is getting bigger, now at 3.6x concentration, and to be safe I'd probably cut that down to 3, but still a 3 gallon tank is less awkward than a 10 gallon tank. On an unrelated note shout out for my type of "chemistry," I see you do drug discovery. Last edited by hwttdz; 07/17/2008 at 02:58 PM. |
|
|

|
|
|
#11 |
|
Reef Chemist
 Join Date: Apr 2001
Location: Arlington, Massachusetts
Posts: 86,233
|
, not that a tank requires sulfate in large quantities,
I disagree. Sulfate is the third most abundant ion in seawater by weight: What is seawater http://reefkeeping.com/issues/2005-11/rhf/index.php from it: 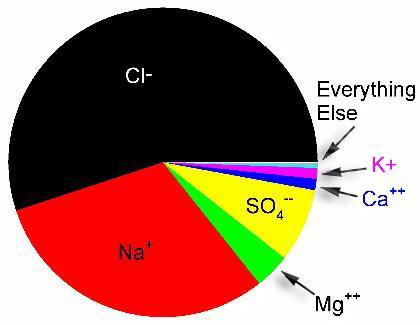 Figure 3. Relative concentration of ions in seawater by weight.
__________________
Randy Holmes-Farley Current Tank Info: 120 mixed reef |
|
|

|
|
|
#12 |
|
Reef Chemist
 Join Date: Apr 2001
Location: Arlington, Massachusetts
Posts: 86,233
|
FWIW, I do not recommend "controlling" salinity in the tank via conductivity. While conductivity is a great way to measure salinity, and is what I use, there is, IMO, to much risk that it will become inaccurate due to deposits on it, bubbles between the electrodes, etc. So I do not recommend leaving the electrode in the water 24/7, or if you do, not allowing the system to respond automatically to a measured "change".
I see you do drug discovery. Yes, I lead the Therapeutic Polymer and Biomaterial Portfolio of research programs at Genzyme. I co-invented these drugs: www.renagel.com www.renvela.com www.welchol.com www.cholestagel.com (European version of Welchol)
__________________
Randy Holmes-Farley Current Tank Info: 120 mixed reef |
|
|

|
|
|
#13 |
|
Registered Member
Join Date: Sep 2007
Location: NY, NY
Posts: 105
|
Tis a good point about not using conductivity to measure salinity.
What do you suppose the effect would be if sulfate were not as high as in sea water? It seems it's just coordinating with positive ions (I'm not that that's not important I'm just saying I don't understand how it is). Also while your plot looks pretty it's by weight, as I assume you know as you made it, and sulfate is quite heavy, so it inflates its importance. So you have no objections using up to a 3.6x concentration, given that at that point there would be zero precipitates? But then again a few pages earlier he indicated 5.6x concentration. I think the experiment would still be informative. 
Last edited by hwttdz; 07/17/2008 at 03:30 PM. |
|
|

|
|
|
#14 |
|
Reef Chemist
 Join Date: Apr 2001
Location: Arlington, Massachusetts
Posts: 86,233
|
so it inflates its importance.
Size isn't everything, as they say.  Phosphate won't even show up on that, and is very important.  FWIW, the article has a similar graph by mole percent.  I do not know what happens with excess or deficiencies of sulfate, since it never happens in he ocean and no one studies it, but it is something that we strive to prevent in other contexts, such as developing a DIY two part system: An Improved Do-it-Yourself Two-Part Calcium and Alkalinity Supplement System http://reefkeeping.com/issues/2006-02/rhf/index.php
__________________
Randy Holmes-Farley Current Tank Info: 120 mixed reef |
|
|

|
 |
| Thread Tools | |
|
|