


|
 |
|
|
#26 |
|
Registered Member
Join Date: Jan 2009
Location: Lubbock, Texas
Posts: 2,174
|
|
|
|

|
|
|
#27 |
|
Registered Member
Join Date: Dec 2010
Posts: 241
|
This is a mystery to me. I agree with Randy for sure, but I also think you've done enough testing with different kits/meters under different conditions and on different tanks to believe the pH and Alk values.
How could CO2 affect this one tank but not the others? Hmmm.... You sure you don't have a soda bottling machine right next to it? Or your skimmer is hooked up to a CO2 tank? Hopefully this is just an odd occurrence and it goes away after several water changes. Good luck. |
|
|

|
|
|
#28 |
|
Registered Member
Join Date: Mar 2008
Location: Davis, Ca.
Posts: 816
|
What if the question (quoted below) is re-stated as:
Could there be something in this tank's water that chemically interferes with the dye-indicators used in the test kits? [QUOTE=... Could there be another dissolved ion in the water causing a low pH besides CO2? Some sort of confounding factor only with my reef? [/QUOTE] |
|
|

|
|
|
#29 |
|
Registered Member
Join Date: Jan 2004
Posts: 2,171
|
Is there a skimmer on your reef tank and no skimmer on the other tank? Or an open top on the reef and closed top on the other? Basically any reason you would have more aeration on the reef than other tank?
The reason I ask is maybe the extra aeration is saturating the water with CO2. On the other hand, the other tank, if there is less gas exchange, maybe is not CO2 saturated. This scenario is actually possible. Another option is the planted tank is consuming a large portion of CO2. This also seems very likely. Could be a combination as well.
__________________
Our imagination is stretched to the utmost, not, as in fiction, to imagine things which are not really there, but just to comprehend those things which are there. ~Richard Feynman |
|
|

|
|
|
#30 | |
|
Registered Member
Join Date: Dec 2010
Location: Memphis, TN
Posts: 91
|
Quote:
The reef has an open top with a fan to cool my LED heatsink and provide surface gas exchange. My suspicion is that you are correct, and the increased aeration of my CO2 saturated home is dropping the pH of my reef. Update: I re-tested the pH of my reef after having all my windows open all day, and I am now at a pH of 7.8. I now am higher than I have been in a couple of months, so I'm pretty sure I'm on the right track. Perhaps with a couple of water changes and more air intake from outside I will be alright. I will probably start dripping kalk at night as well to prevent huge swings in pH. Thanks for all your help guys. |
|
|
|

|
|
|
#31 |
|
Reef Chemist
 Join Date: Apr 2001
Location: Arlington, Massachusetts
Posts: 86,233
|
How could CO2 affect this one tank but not the others?
The relationship is different in fresh water, and if the new RC is not fully aerated with high CO2 home air, then the pH will not drop from where it initially mixes to.
__________________
Randy Holmes-Farley Current Tank Info: 120 mixed reef |
|
|

|
|
|
#32 |
|
Reef Chemist
 Join Date: Apr 2001
Location: Arlington, Massachusetts
Posts: 86,233
|
I re-tested the pH of my reef after having all my windows open all day, and I am now at a pH of 7.8. I now am higher than I have been in a couple of months, so I'm pretty sure I'm on the right track. Perhaps with a couple of water changes and more air intake from outside I will be alright.
I certainly believe pH 7.8 is likely. Water changes are not usually useful for solving pH problems, but are certainly not a problem to do them. FWIW, I think planted tanks often run CO2 deficient, hence the addition of CO2 in such tanks.
__________________
Randy Holmes-Farley Current Tank Info: 120 mixed reef |
|
|

|
|
|
#33 | ||
|
Registered Member
Join Date: Jan 2004
Posts: 2,171
|
Quote:
Quote:
__________________
Our imagination is stretched to the utmost, not, as in fiction, to imagine things which are not really there, but just to comprehend those things which are there. ~Richard Feynman |
||
|
|

|
|
|
#34 | |
|
Registered Member
Join Date: Jan 2004
Posts: 2,171
|
Quote:
Right, Co2 will enter the water quickly, but less quickly if there is less air exchange. In the meantime, the plants are probably consuming the CO2 in the tank. So, I think it's likely the combination. I think kalk and maybe a CO2 scrubbing system, if still too low after the kalk, should help the reef.
__________________
Our imagination is stretched to the utmost, not, as in fiction, to imagine things which are not really there, but just to comprehend those things which are there. ~Richard Feynman |
|
|
|

|
|
|
#35 |
|
Registered Member
Join Date: Oct 2010
Posts: 5
|
What if we aerate the water outside when we are reading high ph as above 8.5? Is it going to decrease?
|
|
|

|
|
|
#36 |
|
Reef Chemist
 Join Date: Apr 2001
Location: Arlington, Massachusetts
Posts: 86,233
|
Depends on the alkalinity, but yes, aerating inside or outside will lower pH when the pH in the water is higher than it should be based on the alkalinity and CO2 level in the air.
I show that there: High pH: Causes and Cures http://reefkeeping.com/issues/2005-03/rhf/index.htm from it: 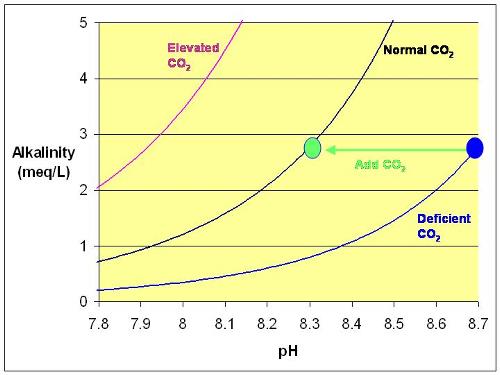 Figure 5. The effect of aeration on alkalinity and pH.
__________________
Randy Holmes-Farley Current Tank Info: 120 mixed reef |
|
|

|
|
|
#37 | |
|
Registered Member
Join Date: Apr 2010
Location: Texas
Posts: 1,532
|
Quote:
|
|
|
|

|
|
|
#38 |
|
Reef Chemist
 Join Date: Apr 2001
Location: Arlington, Massachusetts
Posts: 86,233
|
Yes, that can help.

__________________
Randy Holmes-Farley Current Tank Info: 120 mixed reef |
|
|

|
|
|
#39 | |
|
Registered Member
Join Date: Dec 2010
Location: Memphis, TN
Posts: 91
|
Quote:
|
|
|
|

|
|
|
#40 |
|
Reef Chemist
 Join Date: Apr 2001
Location: Arlington, Massachusetts
Posts: 86,233
|
 Sounds good. Happy Reefing. 
__________________
Randy Holmes-Farley Current Tank Info: 120 mixed reef |
|
|

|
|
|
#41 |
|
Registered Member
Join Date: Feb 2011
Posts: 72
|
Is your low ph tank in your bedroom? I logged CO2 levels in my bedroom and living room over several days. The living room stayed around 450ppm 24/7 while my bedroom at night with the windows closed increased over 1600ppm.
|
|
|

|
|
|
#42 |
|
Reef Chemist
 Join Date: Apr 2001
Location: Arlington, Massachusetts
Posts: 86,233
|
while my bedroom at night with the windows closed increased over 1600ppm.
Probably helps you sleep soundly. 
__________________
Randy Holmes-Farley Current Tank Info: 120 mixed reef |
|
|

|
|
|
#43 |
|
Registered Member
Join Date: Feb 2011
Posts: 72
|
Im surprised im not dead.
|
|
|

|
|
|
#44 |
|
Team RC Member
 Join Date: Aug 2008
Location: Highland, Maryland Entomologist
Posts: 14,591
|
If you have a ventless fireplace this will drive up the CO2 levels in your tank quite a bit. Faulty gas and oil heathers that are not exhausting properly can do this as well.
__________________
Cliff Babcock Intestests: Digital Microscopy; Marine Pest Control; Marine Plants & Macroalgae Current Tank Info: 180 g. mixed reef system |
|
|

|
|
|
#45 |
|
Moved On
Join Date: Nov 2013
Posts: 23
|
I have read that lava rock can lower ph in some cases, do you have any lava rock in your water? I am fighting the same problem... Its beyond me...
|
|
|

|
|
|
#46 |
|
Registered Member
Join Date: Jun 2012
Location: PA
Posts: 2,564
|
I'm hoping he solved his problem, since this post is from 2011.

|
|
|

|
|
|
#47 |
|
Registered Member
Join Date: Dec 2013
Location: Houston,TX
Posts: 198
|
Inexplicable,
Did this resolve your issue? Please respond, i have a similar issue and have done all kind of stuff and think my water is contaminated but will do the aeration measurements as well today to see. My ph is dropping down to 7.4 at an Alk of 8-10. I could no believe that co2 can be so high in the house to drop the ph that low. PSX |
|
|

|
|
|
#48 | |
|
Registered Member
Join Date: Aug 2006
Location: IE
Posts: 1,422
|
Quote:
If you make your own salt I've heard it can also come from the tap water - maybe worth it to test for C02. HTH
__________________
"In a free government the security for civil rights must be the same as that for religious rights." -James Madison Current Tank Info: 120 Visio // Radions // Eschopps Skimmer // MP40 x2 // Tunze ATO // BRS Dosing Pumps and Dual Reactor // Apex Gold // |
|
|
|

|
|
|
#49 |
|
Registered Member
Join Date: Dec 2013
Location: Houston,TX
Posts: 198
|
All right guys,
this thread resolved my issue. In the past I even ran down to 7.2 but mostly 7.5 or so. I did the aeration test today, thanks to this simple and genius method to detect if Co2 in the room affect the reef tank! Took the water from the tank and aerated it within the room. The result was, it dropped to 7.6 then I interrupted the aeration and brought the jar outside and aerated it for around 5-6 hours. Then the Water PH in the jar went up to 7.91. Great!!!! So I brought this jar back into the house and kept aerating it. It dropped again just within 45 minutes down to 7.71. So I ordered the Co2 Absorber and a RODI cartridge at THE FILTERGUYZ. This will be connected to the Air inlet of the Skimmer. Theoretically, the Co2 reduced Air will positively increase the PH just because there is no new Co2 introduced, and as well the aeration of Co2 free air will get the existing Co2 out of the water. Since the Durso's and Water surface is helping the Co2 gettting into the tank as well. I will post the results to see how effective this is gonna be. Happy Reefing PSX  
|
|
|

|
 |
| Tags |
| low ph |
|
|
 Similar Threads
Similar Threads
|
||||
| Thread | Thread Starter | Forum | Replies | Last Post |
| Ph is 7.8, and alkalinity is 1.2 meq/L. How to raise ph and alk? Can I use Bionic alk | cirionrc | Reef Discussion | 1 | 10/18/2010 05:13 AM |
| Ph 7.48-7.88 is LOW...great growth and health still | serum153 | The Reef Chemistry Forum | 17 | 03/31/2010 04:59 AM |
| fw tank - ph problem - 7.2 | dnsfpl | Reef Discussion | 2 | 10/01/2009 09:47 AM |
| New Tank, Ph 7.8 too low? | Thunk | New to the Hobby | 8 | 11/09/2007 05:55 PM |
| pH @ 7.8, too low? | jimrawr | New to the Hobby | 9 | 01/30/2007 04:14 PM |