


|
 |
|
|
#1 |
|
Registered Member
Join Date: Dec 2010
Location: Memphis, TN
Posts: 91
|
pH - is 7.2 too low?
I am getting consistently low (7.2) pH in the morning for the past couple of months now. Throughout the day the pH raises to about 7.6, but never gets higher. To make sure my readings are correct, I borrowed a calibrated lab grade pH meter from one of my labs and it shows the same. SPS polyp extension is great and I am getting good growth rates. I only dose Seachem's Reef Builder and Advantage Calcium to maintain my SPS and my clam. I have two fish (clown and a damsel) and I don't overfeed. No recent deaths in the tank. I have a good skimmer, run a shallow sand bed with a remote DSB in my sump. I do a 15% water change every week with Reef Crystals salt, and pH of the new saltwater is ~8.3. I have a BRS ro/di unit with tds at 0 ppm. I run LEDs with a fan blowing across the surface of the water, so I'm not too concerned with CO2 degassing. I also took a cup of water outside and aerated it to see its effect, and only saw an increase in pH from 7.21 to 7.45, which still seems too low. I have read up as much as I can on pH and the consensus is to let it be what it wants to as long as everything else is in order. What I am curious about is this: is there any evidence of people who run a consistently low pH with success over time? And what could be causing my low pH? My reef is doing great with the exception of the low pH. I donít want to cause undue stress to my inhabitants, however. Any advice is appreciated.
Here are my specs: 20 gallon mixed reef (mushrooms, zoas, leathers, LPS, heavy on SPS, 2 fish, 1 clam) 10 gallon sump/fuge with chaeto in reverse photo-period specific gravity = 1.026 (with refractometer) dKH = 9.0 Ca = 480 ppm Mg = 1350 Phosphates, nitrates, nitrites, ammonia = undetectable temperature = 81 F on controller, never wavers |
|
|

|
|
|
#2 |
|
Registered Member
Join Date: Jul 2007
Location: Orange County CA
Posts: 3,819
|
Measure a cup of water away from the tank.
|
|
|

|
|
|
#3 |
|
Registered Member
Join Date: Dec 2010
Location: Memphis, TN
Posts: 91
|
|
|
|

|
|
|
#4 |
|
Registered Member
Join Date: Mar 2010
Location: Steamwood, IL
Posts: 1,432
|
what do you have for movement in the tank?
also are you tsting with the lights on or off?
__________________
Matt Lions, Groupers and Eels o my! Current Tank Info: Marineland 60Gal ReefReady cube, DIY led, Euroreef Skimmer Ins-80, Mag 9.5 return |
|
|

|
|
|
#5 |
|
Registered Member
Join Date: Jan 2009
Location: Lubbock, Texas
Posts: 2,174
|
I know you used a calibrated lab grade ph probe, but I dont believe that your ph is 7.2 with a dkh of 9.
Either your dkh is wrong or your ph is wrong or you have a serious CO2 problem. |
|
|

|
|
|
#6 |
|
Registered Member
Join Date: Dec 2010
Location: Memphis, TN
Posts: 91
|
For movement, I have two koralia nanos for 850 gph circulation and a Mag 5 return pump at 500 gph, so a total of over 60x turnover.
I was testing both with the main lights on and off. pH is lowest when the lights are off but even at the end of the day the pH is only at 7.6 or so. I know the dKH seems wrong, but I checked with my Salifert test kit, and even dug out my old API alkalinity test kit and got similar results. My main question was whether or not it is sustainable to keep the water at this pH. |
|
|

|
|
|
#7 |
|
Registered Member
Join Date: Jul 2007
Location: Orange County CA
Posts: 3,819
|
Try doing a chemical test and see what it is. I don't believe your pH is that low either. When was the pH probe last changed?
|
|
|

|
|
|
#8 | |
|
Registered Member
Join Date: Dec 2010
Location: Memphis, TN
Posts: 91
|
Quote:
Salifert is approximately 7.4, definitely not 7.7 API is approximately 7.4 also (as low as it will go) I also dug out some old litmus paper and it is about 7.3 in color Just to see, I retested alkalinity with my API test kit and it is showing a dKH of 8. |
|
|
|

|
|
|
#9 |
|
Registered Member
Join Date: Apr 2010
Location: Texas
Posts: 1,532
|
Is it cold in TN? Maybe high co2 levels? Have you tried opening some windows for an hour or 2 and seeing what happens? Maybe you have a leak in your heating system or you are lucky to have very good weather sealing on your house.
|
|
|

|
|
|
#10 |
|
Reef Chemist
 Join Date: Apr 2001
Location: Arlington, Massachusetts
Posts: 86,233
|
I too think the pH is not 7.2. Calibration fluids can be off, etc.
When you aerated the water with outside air, where did you do the pH measurement? outside? Or at the same location, where electrical interference might still be an issue? If the pH is 7.2, it is a huge problem for animals with calcium carbonate skeletons since they will be slowly dissolving away.
__________________
Randy Holmes-Farley Current Tank Info: 120 mixed reef |
|
|

|
|
|
#11 | |
|
Registered Member
Join Date: Dec 2010
Location: Memphis, TN
Posts: 91
|
Quote:
|
|
|
|

|
|
|
#12 |
|
Registered Member
Join Date: Jul 2007
Location: Orange County CA
Posts: 3,819
|
I think kalk would be a better idea. If CO2 is the problem, then a CO2 scrubber might be better in the long run.
|
|
|

|
|
|
#13 | |
|
Registered Member
Join Date: Dec 2010
Location: Memphis, TN
Posts: 91
|
Quote:
|
|
|
|

|
|
|
#14 |
|
Reef Chemist
 Join Date: Apr 2001
Location: Arlington, Massachusetts
Posts: 86,233
|
Try aerating the cup of water inside as well. That will tell you whether you just need more aeration, or whether you need fresher air to be used.
The trees are not pushing up your CO2 level (if anything, they lower it), but indoor cooking with gas, breathing, and any sort of unvented gas heater or fireplace will push up CO2 indoors. Limewater is generally an excellent option for low pH. This has more: Low pH: Causes and Cures http://reefkeeping.com/issues/2004-09/rhf/index.htm and What Your Grandmother Never Told You About Lime http://reefkeeping.com/issues/2005-01/rhf/index.htm
__________________
Randy Holmes-Farley Current Tank Info: 120 mixed reef |
|
|

|
|
|
#15 |
|
bordox
Join Date: May 2008
Posts: 315
|
what will be the reading when aerated the water with outside air whatever ph reading?
__________________
240 g Custom rimless starphire since 2001. |
|
|

|
|
|
#16 |
|
Registered Member
Join Date: Dec 2010
Location: Memphis, TN
Posts: 91
|
Aerating the water indoors leads to a maximum pH of about 7.5, no matter which test method I use. Thanks for the links to the articles Randy. I'll read through those again in more detail, but it seems that I can increase my alkalinity and then maintain it at a higher level with kalk. This should keep me at a higher, more constant pH.
|
|
|

|
|
|
#17 |
|
Reef Chemist
 Join Date: Apr 2001
Location: Arlington, Massachusetts
Posts: 86,233
|
Aerating the water indoors leads to a maximum pH of about 7.5, no matter which test method I use.
If you aerated long enough, then you have quite elevated indoor CO2, or the pH reading is off, or both. iIt should rise above 8.2 for seawater with 9 dKH alkalintiy and sufficient aeration with normal air.
__________________
Randy Holmes-Farley Current Tank Info: 120 mixed reef |
|
|

|
|
|
#18 |
|
Reef Chemist
 Join Date: Apr 2001
Location: Arlington, Massachusetts
Posts: 86,233
|
what will be the reading when aerated the water with outside air whatever ph reading?
Perfectly aerated seawater will follow the blue line on this chart from my article above: 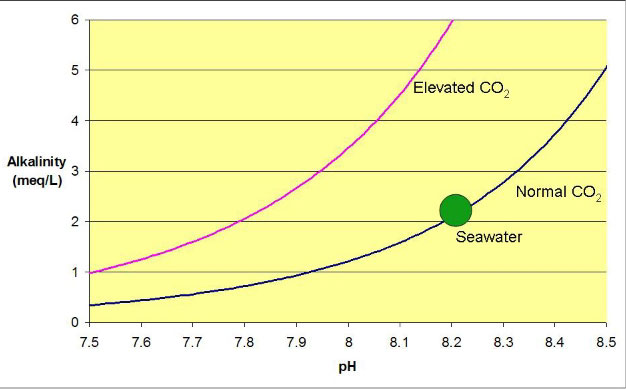 Figure 1. The relationship between alkalinity and pH for seawater equilibrated with air containing normal and elevated carbon dioxide levels. The green dot shows natural seawater equilibrated with normal air, and the curves reflect the result that would be obtained if the alkalinity were artificially raised or lowered.
__________________
Randy Holmes-Farley Current Tank Info: 120 mixed reef |
|
|

|
|
|
#19 |
|
Registered Member
Join Date: Dec 2010
Location: Memphis, TN
Posts: 91
|
I agree with you that the pH should be higher than it is. However, I just now tested newly mixed saltwater here in my house and it is constant at a pH of 8.3. I also tested my wife's saltwater planted tank, which is at a pH of 8.3, and my FOWLR that is at 8.2. It is only my reef tank and the water within it that is low. Could there be another dissolved ion in the water causing a low pH besides CO2? Some sort of confounding factor only with my reef?
I left the cup inside aerating from earlier and just now tested it to be 7.5 after ~45 minutes. I opened all my windows this morning and even set up a box fan to blow in fresh air. We have all electric heating and appliances, no gas lines at all. |
|
|

|
|
|
#20 |
|
Registered Member
Join Date: Jan 2009
Location: Lubbock, Texas
Posts: 2,174
|
Can you check your alk with another test kit?
|
|
|

|
|
|
#21 |
|
bordox
Join Date: May 2008
Posts: 315
|
Randy without you it is so boring
 just a favour. we love rc. but we miss you.
__________________
240 g Custom rimless starphire since 2001. |
|
|

|
|
|
#22 |
|
Registered Member
Join Date: Dec 2010
Posts: 241
|
So putting all your posts together:
1) Your test kits are fine and the reading is correct. 2) CO2 is not the culprit, because the other tanks would be similarly affected. Plus you have a skimmer so that is aerating your tank. So I have to think your water is contaminated with something that is driving down your pH. |
|
|

|
|
|
#23 |
|
Registered Member
Join Date: Dec 2010
Location: Memphis, TN
Posts: 91
|
|
|
|

|
|
|
#24 | |
|
Registered Member
Join Date: Dec 2010
Location: Memphis, TN
Posts: 91
|
Quote:
Just to see, I think I'm going to do 20% water changes each day for the next several days. I already have salt mixed that I have tested to be fine. This should be slow enough to not shock the system too much. I don't want to cause a pH spike. |
|
|
|

|
|
|
#25 |
|
Reef Chemist
 Join Date: Apr 2001
Location: Arlington, Massachusetts
Posts: 86,233
|
Could there be another dissolved ion in the water causing a low pH besides CO2? Some sort of confounding factor only with my reef?
If the carbonate alkalinity is correct, then no, there is no other way pH can be low except elevated CO2 in the water. Any other way of lowering pH must lower alkalinity.
__________________
Randy Holmes-Farley Current Tank Info: 120 mixed reef |
|
|

|
 |
| Tags |
| low ph |
|
|
 Similar Threads
Similar Threads
|
||||
| Thread | Thread Starter | Forum | Replies | Last Post |
| Ph is 7.8, and alkalinity is 1.2 meq/L. How to raise ph and alk? Can I use Bionic alk | cirionrc | Reef Discussion | 1 | 10/18/2010 05:13 AM |
| Ph 7.48-7.88 is LOW...great growth and health still | serum153 | The Reef Chemistry Forum | 17 | 03/31/2010 04:59 AM |
| fw tank - ph problem - 7.2 | dnsfpl | Reef Discussion | 2 | 10/01/2009 09:47 AM |
| New Tank, Ph 7.8 too low? | Thunk | New to the Hobby | 8 | 11/09/2007 05:55 PM |
| pH @ 7.8, too low? | jimrawr | New to the Hobby | 9 | 01/30/2007 04:14 PM |